

Forward
In China, controlled atmosphere heat treatment is becoming increasingly popular, gradually replacing traditional mesh belt furnaces for heating and quenching. This aligns with the trend of industrial upgrading and manufacturing transformation in the country. The automotive industry, in particular, demands ever-higher quality and stricter cost control. Controlled atmosphere heating with minimal oxidation can improve product quality, reduce material loss and machining allowances, leading to both enhanced quality and lower costs.
CQI-9, an early heat treatment system assessment system within the industry, has specific regulations for carbon potential control in a controlled atmosphere. Sections 3.7 and 3.8 of CQI-9 Third Edition clearly define the requirements for carbon potential control, monitoring, recording, and calibration.
Firstly, the carbon potential/dew point (CO2) of the atmosphere in both the atmosphere generator (RX furnace) and the heat treatment furnace should be continuously monitored, automatically controlled, and recorded. The recorded carbon potential should be controlled within ±0.05 of the set value.
Secondly, there should be a backup method for verifying carbon potential/dew point (CO2), such as a dew point meter, hot wire resistor, tri-gas analyzer, foil carbon determination, or carbon rods.
Finally, the carbon potential of the main furnace atmosphere should be verified daily using the backup method, a process known as carbon potential calibration.
Since CQI-9 requires daily carbon potential calibration, a fast, accurate, and convenient verification method is crucial, especially for manufacturers with multiple furnaces. Here's a brief overview of several carbon potential calibration methods:
Dew point meter: Cannot directly measure or calculate carbon potential.
Hot wire resistor: Faster than foil carbon determination, with an optimal carbon potential testing range.
Tri-gas analyzer: Rapid carbon determination, but the test results are influenced by the type of atmosphere and equipment.
Foil carbon determination: The most accurate carbon determination method, but it requires skilled operators and is time-consuming.
Carbon rods: Require composition analysis, making the measurement process complex.
Backgrounds
Although China's automotive industry is developing rapidly and has made significant progress in many areas, there is still a gap between its heat treatment technology and that of developed countries. Taking carburizing control as an example, China is currently popularizing controlled atmosphere technology, while the United States has gradually adopted vacuum carburizing and gas quenching.
A sample survey of 45 heat treatment companies using protective atmosphere heating in the automotive industry showed that the nitrogen-methanol and propane carburizing atmosphere is the most widely used, accounting for 80% (as shown in Figure 1).
(as shown in Figure 1)
Carbon potential calibration predominantly relies on fixed carbon foils, accounting for approximately 90% of applications. Methods like Triple Gas Analyzer and hot wire resistors are less commonly employed (as depicted in Figure 2).
(as depicted in Figure 2)
The frequency of carbon potential calibration deviates significantly from the CQI-9 requirement of daily calibration. Most companies only perform weekly calibrations, while a few calibrate every six months (as shown in Figure 3).
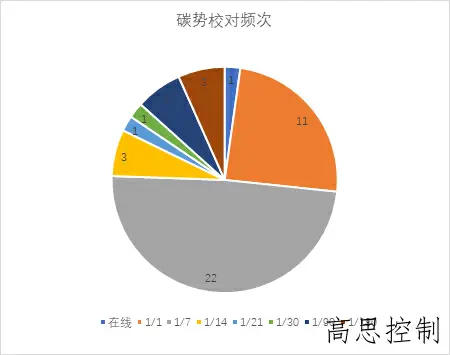
(as shown in Figure 3)
The level of carbon potential control is generally not high. Among the 45 companies surveyed, 55% of them cannot maintain the carbon potential within the set range of ±0.05 (as shown in Figure 4).

(as shown in Figure 4)
In general, there is a significant deficiency in the control of carbon potential during controlled atmosphere heating. The prominent gap compared to CQI-9 requirements lies in the frequency of carbon potential calibration and the level of carbon potential control.
This article will start with the principles of carbon potential control, combined with the actual situation of heat treatment equipment in China, analyze the factors affecting the accuracy of carbon potential control, and provide some solutions for heat treatment carbon potential control measures.
For the vast majority of gas carburizing equipment, the so-called "carbon potential", that is, the ability of the furnace atmosphere to transfer carbon to this material at this temperature, can be calculated by the mv value of the inserted oxygen probe and the given CO% concentration and temperature.
During the controlled gas carburizing process, the following three main reactions occur:
CH4 → [C] + 2H2 (Methane Reaction)
2CO → [C] + CO2 (Boudouard Reaction)
CO + H2 → [C] + H2O (Water-Gas Reaction)
Typically, when designing the atmosphere, the residual methane content after introducing the carrier gas is kept minimal. This brings the atmosphere close to chemical equilibrium and maintains stability. The carburizing process is then driven by the equilibrium established by the Boudouard and water-gas reactions, involving CO, H2, CO2, and H2O:
CO + H2O ↔ CO2 + H2 (Continuous Water-Gas Shift Reaction)
Under these specific conditions, the carbon transfer coefficient can be effectively characterized by analyzing either the water-gas reaction or the reactions occurring at the material's surface. This analysis subsequently provides valuable insights into the prevailing carbon potential.
According to the German standard DIN 17022, Part 3, the carbon transfer coefficient ac:
The unit of gas partial pressure is Bar; the temperature is in Kelvin; the formula for converting to carbon potential (CP) is:
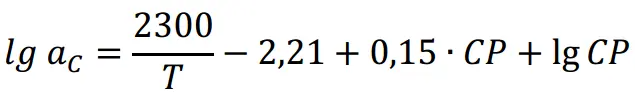
Based on the redox relationship of CO, H2, CO2, and H2O, the conversion can be substituted into the equation as the partial pressure of oxygen:
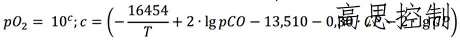
(Formula 1)
In a pre-designed "relatively stable atmosphere," we calculate and control the carbon potential of the furnace atmosphere through real-time temperature, oxygen partial pressure signals, and a given CO concentration (preset value).
3.1 Understanding Oxygen Probe Functionality in Heat Treatment Processes
The oxygen probe, as depicted in Figure 5 (schematic diagram), consists of a closed ceramic tube made of zirconium oxide. Zirconium oxide has the unique property of being permeable to oxygen ions. When a difference in oxygen partial pressure exists between the inner and outer surfaces of the zirconium oxide tube, a voltage potential is generated, which can be measured using a millivoltmeter.
To utilize this characteristic of zirconium oxide for measuring the oxygen partial pressure within a furnace, we need a reference point, which is the oxygen partial pressure on one side of the zirconium oxide tube. Generally, we leverage the relatively stable oxygen content (oxygen partial pressure) in the air to establish a stable reference oxygen partial pressure inside the zirconium oxide tube.
This is achieved by using an air pump to introduce reference air containing 20.9% oxygen into the inner tube. The difference in oxygen content between the measured atmosphere and the reference air generates a voltage potential. This potential is continuously measured through the platinum metal electrode (inner electrode) inside the zirconium oxide tube and the outer heat-resistant steel tube (outer electrode). The resulting measurement is the mV value output by the zirconium oxide probe.
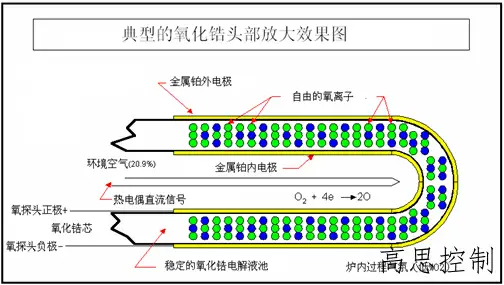
(as shown in Figure 5)
The relationship between the electromotive force (EMF) in millivolts (mV) of an oxygen probe and oxygen partial pressure can be characterized by the Nernst Equation.:

EMF (mV); T (Kelvin temperature); PO2 (Bar), substitute the oxygen partial pressure into the above formula 1 to obtain:
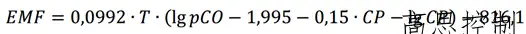
(Formula 2)
Where: EMF represents the oxygen probe's millivolt value; T denotes the atmosphere temperature; PCO signifies the partial pressure of CO in the atmosphere (can be expressed as CO% volume fraction); CP stands for the carbon potential of the atmosphere.
As can be seen from the above, the main factors affecting carbon potential calculations are:
oxygen probe output status;
furnace temperature accuracy;
atmosphere stability (CO% concentration).
The furnace temperature uniformity of a conventional controllable atmosphere heat treatment equipment at 3.2°C can reach within ±5°, with carburizing at 930°C, CO%=20.00%, and CP=1.20. The influence of a 5°C furnace temperature difference on the carbon potential is shown in Figures 6 and 7.
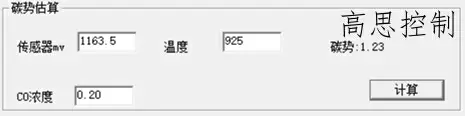
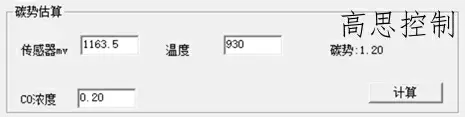
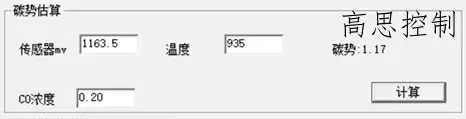
(as shown in Figure 6)
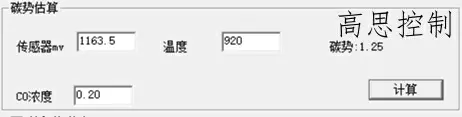
(as shown in Figure 7)
As can be observed, in the high-temperature segment, a deviation of ±5°C can lead to a carbon potential deviation of at least ±0.03.
A deviation of ±10°C can lead to a carbon potential deviation of at least ±0.05.
860°C; CO = 30.00%; CP = 1.0; The influence of a 5°C furnace temperature difference on the carbon potential is shown in Figures 8 and 9
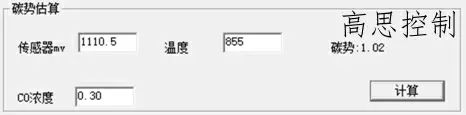
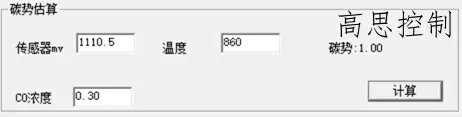
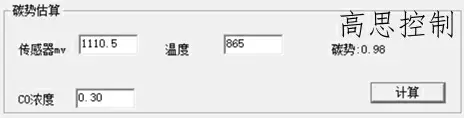
(as shown in Figure 8)
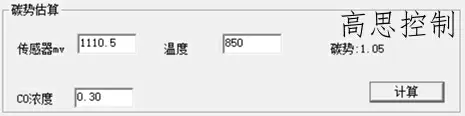
(as shown in Figure 9)
As can be seen, in the low-temperature section, a deviation of ±5° can lead to a carbon potential deviation of at least ±0.02.
A deviation of ±10° can lead to a carbon potential deviation of at least ±0.05.
This shows that regular furnace temperature uniformity tests and thermocouple accuracy tests on heat treatment equipment are the basis for ensuring carbon potential control.
3.2 Proper Use and Maintenance of Oxygen Probes.
Oxygen probes are undoubtedly one of the most effective and economical sensors for carbon potential control. Correct selection, maintenance, and care of oxygen probes can effectively reduce the risk of carbon potential runaway.
Currently, there are four main types of zirconia probes on the market, as shown in Figure 10.

(as shown in Figure 10)
Explanation of Different Oxygen Probe Designs:
1、Full Zirconia Design:** This design likely refers to an oxygen probe where the sensing element and other major components are made entirely of zirconia.
2、Zirconia Ball Design:** This could refer to a design where a zirconia ball acts as the sensing element or a key component within the probe.
3、Zirconia-Ceramic Welding Design:** This likely describes a probe where zirconia and ceramic components are joined together using a welding process.
4、Zirconia Tip Design:** This design probably refers to a probe with a sensing tip made of zirconia.
Whether it is a full zirconia design, a zirconia ball design, a zirconia-ceramic welding design, or a zirconia tip design, ensuring a good seal between the zirconia and the ceramic inner tube is crucial for any commercially available oxygen probe. Typically, the output mV error of a new oxygen probe needs to be guaranteed within ±5 mV.
In a typical carburizing process, a deviation of ±5 mV can lead to a significant carbon potential deviation, as shown in Figure 11.
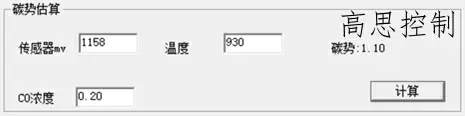
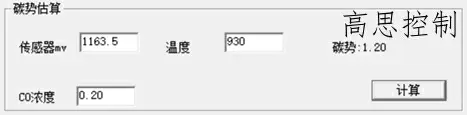
(as shown in Figure 11)
(When carburizing at 930°C, a 5mv attenuation of the oxygen probe affects the carbon potential by: ±0.10CP)
Maintaining entry and exit records of oxygen probes; performing routine inspection records; and replacing oxygen probes promptly at appropriate times are effective means to ensure stable carbon potential control.
3.3 Carrier Gas (Protective Atmosphere)
Controlled atmosphere heat treatment furnaces primarily utilize carrier gases to establish carbon potential equilibrium and adjust the carbon potential through enriching agents. Therefore, a stable and reliable protective atmosphere is a prerequisite for carbon potential control.
3.3.1 Endothermic Atmosphere:
The endothermic atmosphere is the earliest controlled equilibrium atmosphere used. It is generated by the endothermic gas generator, which directionally cracks air and natural gas under the action of a high-temperature catalyst, "transforming" them into cracked gas (RX gas), as shown in Figure 12.
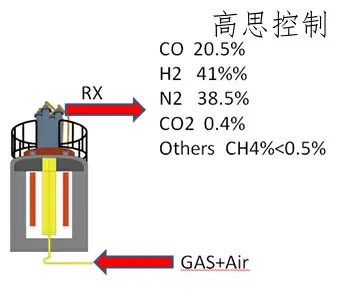
(as shown in Figure 12)
Cracked gas, primarily composed of CO, H2, N2, and trace amounts of CO2, is widely used as a carrier gas in carburizing and hardening processes, as well as a protective gas in annealing processes.
Its directional cracking equation is:
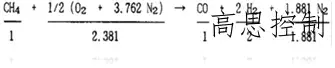
(Formula 3)
RX gas, as a controlled atmosphere, boasts stable composition and excels at providing a relatively constant CO% ratio, which is beneficial for conventional carbon potential control. As the directional cracking process is endothermic, the required energy is supplied by the reforming furnace. This ensures that the cracked gas, upon introduction into the heat treatment furnace, does not affect the control of the internal temperature field.
3.3.2 Methanol Cracking Atmosphere. Liquid methanol is directly dripped into the furnace. After methanol cracking, the concentration of CO% varies between 27% and 31.5% depending on the temperature.
CH3OH → CO + 2H2
Theoretically, after complete methanol cracking, 33.3% CO + 66.7% H2 will be generated. However, due to the incomplete cracking of methanol at lower temperatures, the actual cracked components will vary depending on the temperature and the type of heat treatment furnace.
3.3.3 Nitrogen-methanol atmosphere.
The so-called nitrogen-methanol atmosphere refers to the direct introduction of a fixed ratio of nitrogen and liquid methanol into the furnace. The liquid methanol cracks at high temperatures, producing methanol cracking gas, which simulates the gas composition of an endothermic atmosphere.
When only methanol is introduced into the furnace, the atmosphere composition is: 33.3% CO + 66.7% H2. In practical applications, the basic composition of the nitrogen-methanol atmosphere is adjusted by changing the N2/CH3OH ratio according to process requirements. The 40/60 ratio is the most commonly used nitrogen-methanol atmosphere.
CH3OH → CO + 2H2
After high-temperature cracking of methanol, the volume expansion coefficient is: 1L/H (liquid) is approximately equal to 1.66M3/H (gas). The most commonly used ratio: 1.1m3 of N2 + 1 liter of CH3OH. The main gas composition is: 20% CO + 40% H2 + 40% N2, which is also the 1.1:1 relationship between nitrogen and methanol that we often mention.
Practice has proven that the carbon transfer efficiency of the three atmospheres is different. Due to the higher concentration of CO in the methanol cracking gas, the carbon transfer efficiency is higher and the penetration rate is faster. However, at the same time, the carburizing process also becomes more aggressive and difficult to control. If the carbon transfer efficiency of RX atmosphere is set to 1.0, then the comparison of the penetration rates of the three atmospheres is as follows:
RX Gas by Natural gas (20% CO, 40% H2) coefficient = 1.0
Nitrogen – Methanol (20% CO, 40% H2) coefficient = 1.0
RX Gas by Propane (23% CO, 31% H2) coefficient = 0.9
Pure Methanol (33% CO, 67% H2) coefficient = 2.7
In practical applications, the composition of RX atmosphere and nitrogen-methanol atmosphere is analyzed using a three-component gas analysis system as follows:
Example 1, using RX gas generator to prepare protective gas (RX GAS), 850° empty furnace for carbon determination; CP=0.80;
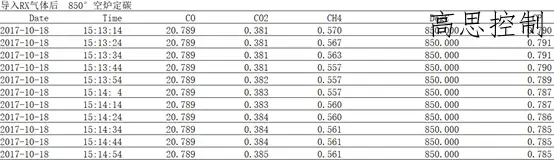
Simultaneously measure the RX as it transforms into furnace components:
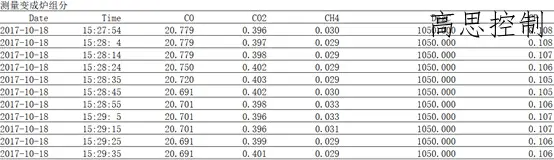
Based on the data above, we can observe the following:
Example 2: Monitoring data of a nitrogen-methanol atmosphere multi-purpose furnace; 920°C / CP=1.1

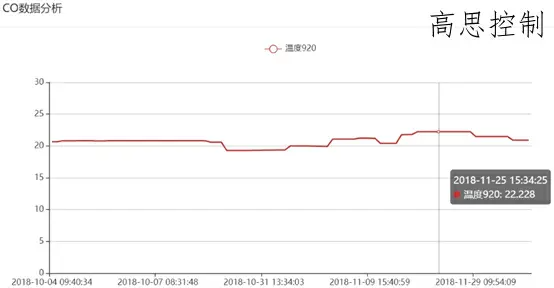
Based on one month of monitoring data, the CO variation range is 19.3% to 22.2%.
This fluctuation causes a carbon potential deviation of ±0.12 CP.
The concentration of CH4 is much greater than that of the RX atmosphere:

Through the comparison of two atmospheres, it can be found that the cracked gas generated by the controllable atmosphere RX gas turning furnace is more stable than the nitrogen-methanol atmosphere, and its carbon potential is easier to control.
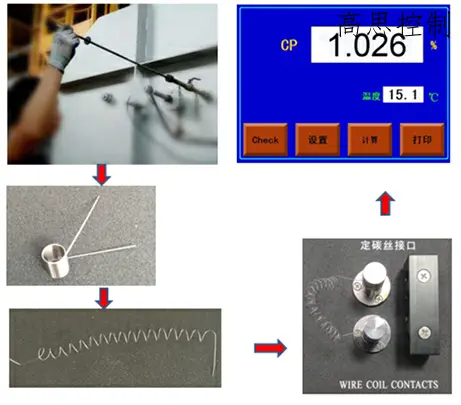
4.1 Resistance wire carbon potential determination.
This method and its associated instruments have been widely used in recent years for carburizing processes in the automotive components industry. Its key components and core algorithms have been fully localized within China. It is a relatively efficient and rapid method for carbon potential determination. The principle involves using a resistance wire made of special materials. After undergoing carburization in the furnace atmosphere, the electrical resistivity of the wire is measured, which in turn allows for the measurement of carbon potential.
Features:
1. Convenient Carbon Potential Measurement: After carburization, direct carbon potential measurement is possible, offering ease of use.
2. High Accuracy: In processes with a carbon potential between 0.7 and 1.2, the measurement accuracy can reach ±0.03.
3. Uninfluenced by Cleaning and Drying: Cleaning and drying processes for the fixed carbon wire do not affect the measurement results, making it less demanding on the operator's skill.
4. Simultaneous Comparison: It can be placed in the furnace together with a fixed carbon sheet, allowing for simultaneous carbon potential comparison.
The operation flow is shown in Figure (13):
4.2 Three-Gas Analysis Method.
This method and its associated instruments have been widely adopted in recent years for carburizing processes in the automotive parts industry. Its key components and core algorithms have been fully localized in China, making it a highly effective and rapid carbon potential determination technique. The primary principle involves real-time measurement of CO% and CO2% along with the given furnace temperature to calculate the carbon potential within the furnace. Simultaneously, it can also measure residual CH4 to monitor the atmosphere quality.

Its main features are:
1. Fast carbon determination results: Typically within 5 minutes;
2. Simple calibration method with third-party accuracy reports: Ensuring reliability and ease of use;
3. Wide applicability to various furnace types and processes: Facilitating easy adoption and implementation;
4. Simple operation process with low requirements for operators: Minimizing the need for extensive training;
5. No consumables, resulting in low usage and maintenance costs: Offering cost-effective operation.

5. Conclusion
To meet the requirements of the automotive industry for controlled atmosphere carbon potential, focusing on the key factors affecting carbon potential, selecting the correct carbon potential calibration method, and increasing atmosphere monitoring and control measures can effectively improve the level of carbon potential control in heat treatment enterprises.
References:
1. 《Heat Treatment Process and Principle》 - Hou Xuming
2. 《Chang'an Ford CQI-9 Training Materials》 - Zhang Shaoyong


